The U.S. Food and Drug Administration (FDA) has issued a guidance, “Supply-Chain Program Requirements and Co-Manufacturer Supplier Approval and Verification for Human Food and Animal Food: Guidance for Industry,” designed to give certain co-manufacturers more time to meet supplier approval and verification requirements under three FDA food safety regulations. These regulations were created under the Food Safety Modernization Act (FSMA) and include the Preventive Controls for Human Foods, Preventive Controls for Animal Food, and the Foreign Supplier Verification Programs. These acts have requirements for a supply-chain program for raw materials and other ingredients and was designed to control any hazards through the implementation of a supply chain. View the guidance here.
FSMA Update: “Co-Manufacturers” Allowed Additional Time to Implement Certain Supply-Chain Program Requirements
Nov 06, 2017 | Amy Chang
Related Posts

- Community Health Community Resilience COVID-19 Performance Improvement
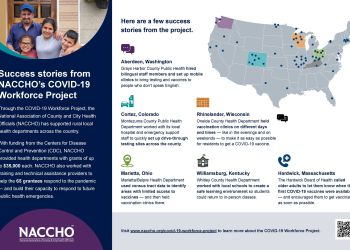
NACCHO’s COVID Workforce Project: Leveraging Community...
At Oneida County Health Department, cooperation is baked into daily operations;...
Apr 11, 2024 | Andrea Grenadier