On July 13, 2022 the U.S. Federal Drug Administration (FDA) issued an emergency use authorization (EUA) for Novavax COVID-19 Vaccine, Adjuvanted for the prevention of the virus in individuals 18 years and older. As with all of the vaccines that received EUA, the process entailed a thorough review of the available data from vaccine manufacturer by the FDA and Advisory Council on Immunization Practices (ACIP).
The Novavax vaccine utilizes vaccine technology that has been utilized in previous vaccines, including human papillomavirus (HPV) and hepatitis B vaccines. The vaccine works differently from the messenger RNA (mRNA) vaccines by injecting only the spike protein of the virus. Novavax also uses an adjuvant, which is an immune stimulant that encourages a better immune response. This technology has been around longer and used in other vaccines, because of this the Novavax vaccine may help to address vaccine hesitancy. Those who have yet to get vaccinated because of worries about the newness of mRNA vaccines may be more accepting of this protein-based vaccine.
The schedule for the virus is as follows: Adults 18+ should receive 2 doses in the primary series, given 3–8 weeks apart
Resources
Novavax Factsheet for Patients
Novavax Factsheet for Providers
The COVID-19 Vaccine Lineup
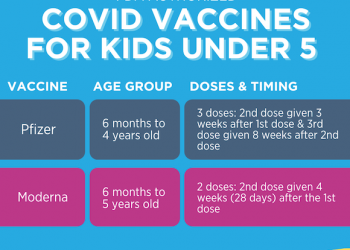
With the authorization of Novavax, there is a now a lineup of vaccines that includes: Moderna, Pfizer, Novavax, and Johnson & Johnson vaccines. There are vaccination options for everyone aged 6 months and older. Each vaccine has varying age ranges, doses, and recommendations. Stay up to date with Your COVID-19 vaccines by checking in on the recommendations and making sure you received your booster doses.
Resources for COVID-19 Vaccines
Scheduling Your COVID-19 Vaccines: To find COVID-19 vaccine locations near you search vaccines.gov, text your ZIP code to 438829, or call 1-800-232-0233.