Popular Categories
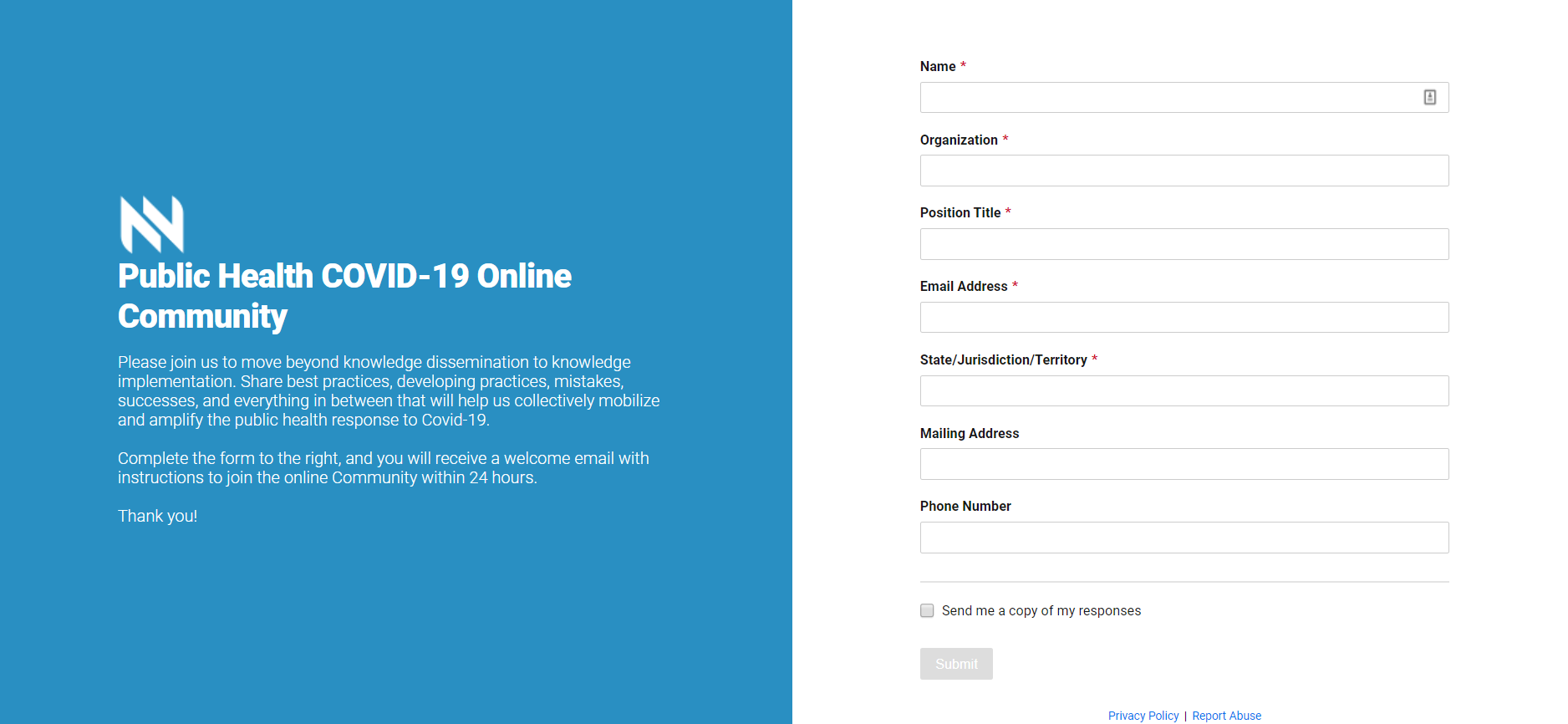
Become a Member of NACSD and NACIDD
The National Advisory Committee on Individuals with Disabilities and Disasters (NACIDD) and National Advisory Committee on Seniors and...
Jul 02, 2021 | Beth Hess
COVID-19
EUA updates for bam/ete and REGEN-COV
The FDA issued major updates to the Emergency Use Authorizations (EUA) for bamlanivimab and etesevimab administered together and...
May 18, 2021 | Beth Hess
Tools & Resources, COVID-19, Medical Reserve Corps
COVID-19 Response Mission Sets
Throughout the COVID-19 Pandemic, Medical Reserve Corps (MRC) units have been adapting and supporting an unprecedented number and type...
Jan 21, 2021 | Beth Hess
COVID-19, Immunization
Pfizer Covid-19 Vaccine Standing Orders
Pfizer-BioNTech COVID-19 Vaccine Standing Orders for Administering Vaccine to Person 16 Years of Age and Older are now available.
Dec 17, 2020 | Beth Hess
Tools & Resources, COVID-19, Immunization
CDC Launches V-safe Website
The CDC has launched V-safe, a smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins...
Dec 15, 2020 | Beth Hess
Tools & Resources, COVID-19, Immunization
New Educational Website: COVID Vaccine Facts
The Biotechnology Innovation Organization (BIO) has launched a new educational website, COVID Vaccine Facts.
Dec 04, 2020 | Beth Hess
Tools & Resources, Contact Tracing, COVID-19
New Recommendations for Prioritizing Case Investigations and Contact Tracing for...
CDC released new recommendations prioritizing case investigations and contact tracing to assist health departments experiencing...
Nov 23, 2020 | Beth Hess
OpportunityBecome a Member of NACSD and NACIDDThe National Advisory Committee on Individuals with Disabilities and Disasters (NACIDD) and National Advisory Committee on Seniors and Disasters (NACSD) is currently accepting applications from non-federal individuals with the expertise needed to provide diverse perspectives on issues critical to these populations and disasters to fill seven new voting member positions within each committee Jul 02, 2021 | Beth Hess |
COVID-19EUA updates for bam/ete and REGEN-COVThe FDA issued major updates to the Emergency Use Authorizations (EUA) for bamlanivimab and etesevimab administered together and REGEN-COV, both authorized for the treatment of mild to moderate COVID-19 in eligible patients. May 18, 2021 | Beth Hess |
Tools & Resources, COVID-19, Medical Reserve CorpsCOVID-19 Response Mission SetsThroughout the COVID-19 Pandemic, Medical Reserve Corps (MRC) units have been adapting and supporting an unprecedented number and type of responses. Many units are using NACCHO’s mission set template to plan and document those response types. Jan 21, 2021 | Beth Hess |
COVID-19, ImmunizationPfizer Covid-19 Vaccine Standing OrdersPfizer-BioNTech COVID-19 Vaccine Standing Orders for Administering Vaccine to Person 16 Years of Age and Older are now available. Dec 17, 2020 | Beth Hess |
Tools & Resources, COVID-19, ImmunizationCDC Launches V-safe WebsiteThe CDC has launched V-safe, a smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins after an individual receives a COVID-19 vaccination. Dec 15, 2020 | Beth Hess |
Tools & Resources, COVID-19, ImmunizationNew Educational Website: COVID Vaccine FactsThe Biotechnology Innovation Organization (BIO) has launched a new educational website, COVID Vaccine Facts. Dec 04, 2020 | Beth Hess |
Tools & Resources, Contact Tracing, COVID-19New Recommendations for Prioritizing Case Investigations and Contact Tracing for COVID-19 in High Burden JurisdictionsCDC released new recommendations prioritizing case investigations and contact tracing to assist health departments experiencing escalating COVID-19 cases. Nov 23, 2020 | Beth Hess |

Subscribe Today
Sign Up for the E-mail Digests
Create an account or login to MyNACCHO and go to "My Subscriptions."
SUBSCRIBE NOW